Ripe blueberry fruit vary widely in perceived firmness depending on cultivar and species, and consumers pick up on these differences. Our consumers want fresh market blueberries to be consistent from berry to berry in both firmness and flavor. Since blueberry sugars do not increase after harvest, the fruit must be harvested near or at full ripeness. Additionally, blueberry firmness usually decreases with storage, but the rate of loss can depend on the cultivar as well as storage temperature.
Introduction
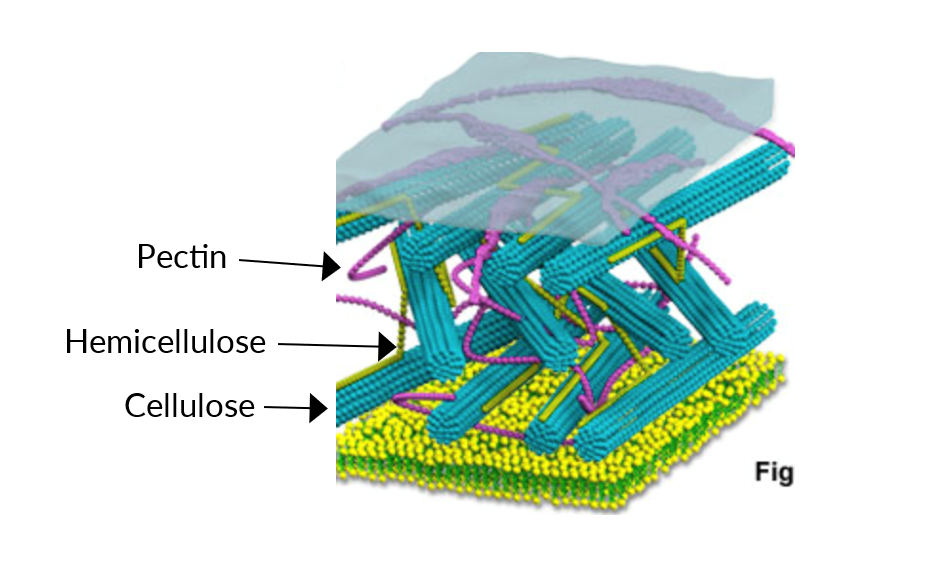
Several factors have been related to blueberry phenotypic firmness such as fruit morphology, turgor pressure, enzyme activity, internal cell structure, and cell wall changes. We know that plant cell walls are highly complex and can differ in both types and amounts of polysaccharides (cellulose, hemicellulose, and pectin) (Figure 1). Changes and variability in fruit firmness have been linked to cell wall polysaccharides via three main causes: 1) loss of cell wall polysaccharides, 2) changes in cell wall neutral sugar composition and 3) breakdown of the middle lamella.
It has been challenging to link blueberry cell wall composition to perceived firmness traits. Total cell wall polysaccharides have not been well correlated with blueberry firmness, and both the peel and pulp of a blueberry can contribute to firmness. Although the types of cell wall polysaccharides and their linkages are important, establishing these relationships is laborious and difficult. Understanding the quantitative cell wall sugar composition and polysaccharide linkage residues in blueberry peel and pulp is needed to understand how polysaccharides are arranged and find how these cellular level molecules contribute to blueberry firmness and texture.
In this study, blueberry cultivars representing firm, soft, and crisp phenotypes were used. Based on other published research, we predicted that crisp and firm blueberries would have more hemicellulose and cellulose, as these polysaccharides are less likely to depolymerize during ripening. The objective of our work was to study the quantitative cell wall differences between the peel and pulp of three highbush blueberry cultivars at harvest.
Selection of the cultivars
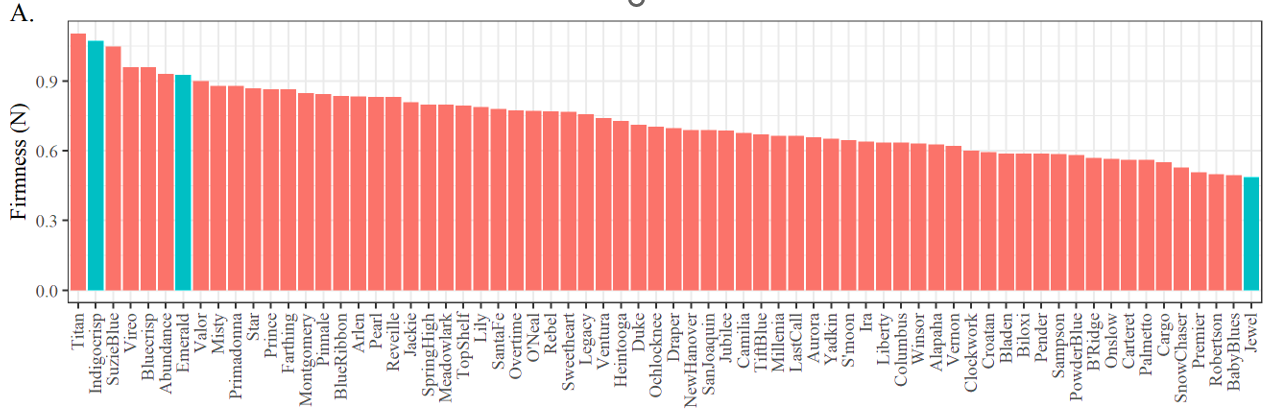
Using 2020 firmness/texture data for blueberry fruit, we found clear firmness differences among crisp, firm and soft cultivars. We selected ‘Indigocrisp’ (crisp), ‘Emerald’ (firm, industry standard), and ‘Jewel’ cultivars (soft) for this study (Figure 2, marked in blue).
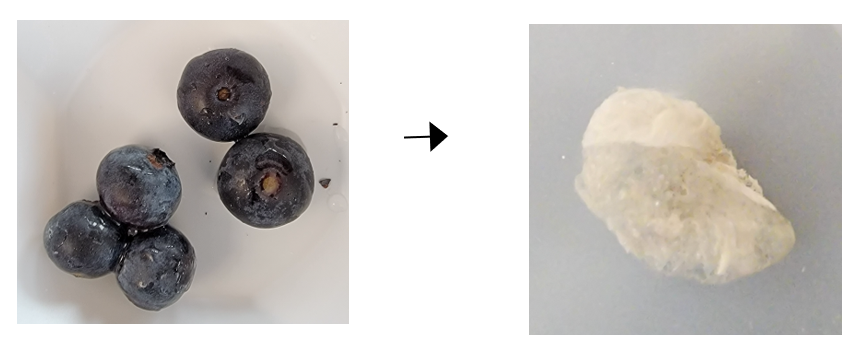
Preparation of peel and pulp cell wall tissue
Frozen whole blueberries of ‘Indigocrisp’, ‘Emerald’, and ‘Jewel’ were packed into test tubes with six steel balls and cold ethanol. Tubes were immersed in liquid nitrogen (N) then samples were ground to detach peel particles from intact pulp. After peel separation, pulp was removed then transferred to a new set of test tubes containing a second set of steel balls. Pulp samples were flash frozen in liquid N and further ground. Then, hot water extraction of the blueberry peel and pulp was done using a series of organic solvents to yield peel and pulp cleaned cell wall tissues (Figure 3).
Quantitative cell wall extraction of blueberry peel and pulp
In order to quantitate cell wall neutral sugars and linkage residues, extensive wet chemistry was performed following Trandel et al. (2020) and Pettolino et al. (2012). All samples went through a series of carboxyl reductions, methylation for linkage residue quantitation, cell wall hydrolysis, derivatization and acetylation and then were ran on a GC-MS. Nuetral sugars of glucose, galactose, arabinose, ribose, xylose, mannose, fucose and rhamnose were quantitated using sugar calibration curves. Uronic acids were calculated and cell wall linkage residues were identified using partially methylated sugar standards and mass spectral data.
Linkage analysis and polysaccharide classification
Linkage analysis is a critical component of quantitative cell wall work as it allows for the determination and estimation of specific polysaccharide classes. Linkage analysis provides information about how the single sugar units (monosaccharides) are linked together through chemical bonds. For example, linkage analysis showed us that carbon 1 on rhamnose was linked to carbon 4 on glucose. By knowing the various linkage types, we can piece together the polysaccharide classes. We can then estimate the amount of specific polysaccharides by looking at the relative proportion of linkages within a polysaccharide. We used the classification calculations provided by Pettolino et al. (2012), who used Arabidopsis as the model crop.
Key results and discussion
Neutral sugar composition
Hemicelluloses were found in the peel and pulp of all three blueberry cultivars, although the type of hemicellulose appears to differ with location and cultivar. ‘Indigocrisp’ peel was high in glucuronic acid and contained xylose. In contrast, ‘Jewel’ peel contained more mannose and arabinose. Arabinose is used to build hemicelluloses like arabinan and type I arabinogalactan, while mannose has been linked to heteromannan and galactoglucomannan (Mariettes et al., 2021; Dheilly et al., 2016). The pulp of ‘Emerald’ blueberries had the most glucose, which is predominantly found in hemicellulosic xyloglucans (Amos et al., 2019).
Unfortunately, these neutral sugar assessments did not fully explain the phenotypic texture differences among cultivars. Identification of cell wall linkage residues was pursued in order to determine the cell wall polysaccharide classes.
Blueberry cell wall linkage residues and differences in pulp and peel among cultivars
In this experiment a total of 45 linkage residues were identified in blueberry peel and pulp. Using this method, ‘Indigocrisp’ pulp was found to be higher in hemicellulose cell wall neutral sugars and linkage residues of xyloglucan, heteromannan, and arabinan. In several fruit crops, hemicelluloses have been most correlated with increased firmness phenotypes, and the type of hemicellulose differentiates crisp and firm phenotypes. In contrast, ‘Jewel’ pulp was high in type I arabinogalactan, and the linkages identified indicated a high percent methylation. Higher percent methylation has been previously related to increased softening during crop storage (Lurie et al., 2003), suggesting cell wall interconnections between arabinogalactan and pectin may degrade at a faster rate in phenotypically soft blueberry cultivars, leading to decreased firmness.
In blueberry peel, ‘Indigocrisp’ had lower concentrations of heteromannan and xyloglucan than the peel of the other cultivars. However, ‘Indigocrisp’ peel had the highest amounts of heteroxylan and this polysaccharide has been associated with increased firmness in eggplant and apple (Mariettes et al., 2016).
The peel of ‘Jewel’, the softer cultivar, had higher concentrations of type I arabinogalactan and heteromannan. Although rhamnogalacturonan amounts did not differ in ‘Jewel’ peel relative to ‘Indigocrisp’ or ‘Emerald’, the increased ratio of Ara/Rha suggests an increased presence of hairy side branches, which could lead to increased softening due to pectin’s susceptibility to depolymerization.
How does phenotypic blueberry firmness relate to cell wall polysaccharides?
Although blueberry cultivars vary greatly in fruit firmness, the underlying causes of this variation have been difficult to discern in previous cell wall studies. In our study, we found the pulp of the crisp cultivar ‘Indigocrisp’ had higher amounts of hemicelluloses, specifically arabinan, heteromannan and xyloglucan, and cellulose, than ‘Emerald’ or ‘Jewel’. These finding suggest increased hemicellulose content in the pulp may lead to higher phenotypic firmness in blueberry.
Literature Cited
Amos, R. A.; Mohnen, D. Critical review of plant cell wall matrix polysaccharide glycosyltransferase activities verified by heterologous protein expression. Front. Plant Sci. 2019, 10, 1-2. doi: https://doi.org/10.3389/fpls.2019.00915
Dheilly, E.; Le Gall, S.; Guillou, M.-C.; Renou, J.-P.; Bonnin, E.; Orsel, M.; Lahaye, M. Cell wall dynamics during apple development and storage involves hemicellulose modifications and related expressed genes. BMC Plant Biol. 2016, 16, 201-209. doi: https://doi.org/10.1186/s12870-016-0887-0
Lurie, S.; Zhou, H. W.; Lers, A.; Sonego, L.; Alexandrov, S.; Shomer, I. Study of pectin esterase and changes in pectin methylation during normal and abnormal peach ripening. Physiol. Plant. 2003, 119, 287-294. doi: https://doi.org/10.1034/j.1399-3054.2003.00178.x
Mariettes, A.; Kang, H. S.; Heazlewood, J. L.; Persson, S.; Ebert, B.; Lampugnani, E. R. Not just a simple sugar: arabinose metabolism and function in plants. Plant Cell Physiol. 2021, 62, 1791-1812. doi: https://doi.org/10.1093/pcp/pcab087
Pettolino, F. A.; Walsh, C.; Fincher, G. B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 9, 1590-1607. doi: https://doi.org/10.1038/nprot.2012.081
Trandel, M. A.; Johanningsmeier, J.; Schultheis, J.; Gunter, C.; Perkins-Veazie, P. Cell wall polysaccharide composition of grafted ‘Liberty’ watermelon with reduced incidence of hollow heart. Front. Plant Sci. 2021, 12, 1-19. doi: https://doi.org/10.3389/fpls.2021.623723
Feature Article by Dr. Marlee Trandel-Hayse, Department of Horticulture, Auburn University













